NP-1
Oral Dosage Treatment for Retinitis Pigmentosa
Retinitis pigmentosa (RP) refers to a group of inherited retinal diseases causing retinal degeneration and vision loss that worsens over time. Often diagnosed in childhood or adolescence, retinitis pigmentosa involves nearly 3100 mutations in more than 50 genes.27 Forms of RP and related diseases include Usher syndrome,28 Leber’s congenital amaurosis, rod-cone disease, Bardet-Biedl syndrome and Refsum disease.

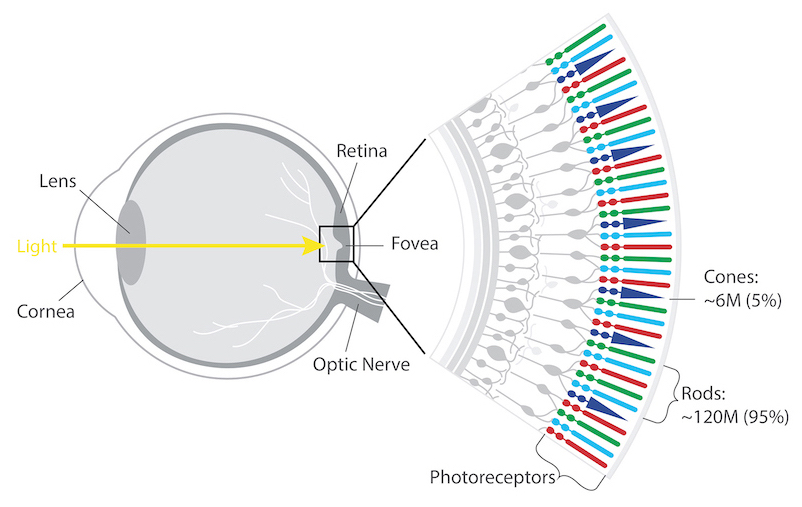
The retina’s photoreceptors (rods & cones) convert light into neural signals and send them to the brain for visual recognition.
Rods are responsible for peripheral, night, and black and white vision.
Cones, centrally concentrated, provide color and central vision.
People with RP experience a gradual decline in their vision because photoreceptor cells (rods and cones) die. In most forms of RP, rods are affected first. Because rods are concentrated in the outer portions of the retina and are triggered by dim light, their degeneration affects peripheral and night vision. Night blindness is one of the earliest and most frequent symptoms of RP. When the more centrally located cones - responsible for color and sharp central vision - become involved, people lose color perception and central vision. Typically diagnosed in adolescents and young adults, RP is a progressive disorder; most people are legally blind by age 40. However, the rate of progression and degree of visual loss varies from person to person.
The National Eye Institute estimates that approximately 100,000 people in the United States have RP. Worldwide, about 1.5 million people are afflicted. There is one approved gene therapy for a small subset of RP. LUXTURNA® is indicated for the treatment of patients with RPE65 mutations, about 1-6% of RP patients.29 While gene therapy has the potential to address pathogenic mutations, with thousands of mutations already associated with RP, a relatively small number of patients are likely to benefit from any single gene therapy. An effective gene-agnostic treatment like NPI-001 has the potential to work alone or in concert with gene therapies to improve patient outcomes.
NPI-001 is a small molecule treatment developed to slow or halt disease progression in RP patients with functioning cones, regardless of their disease-causing genetic mutation. Nacuity’s proprietary, GMP-grade formulation of NPI-001 tablets target oxidative stress associated with blinding eye diseases, such as RP. NPI-001 boosts glutathione, our body’s powerful endogenous antioxidant, to stop chemically aggressive oxygen molecules from damaging eye tissue.
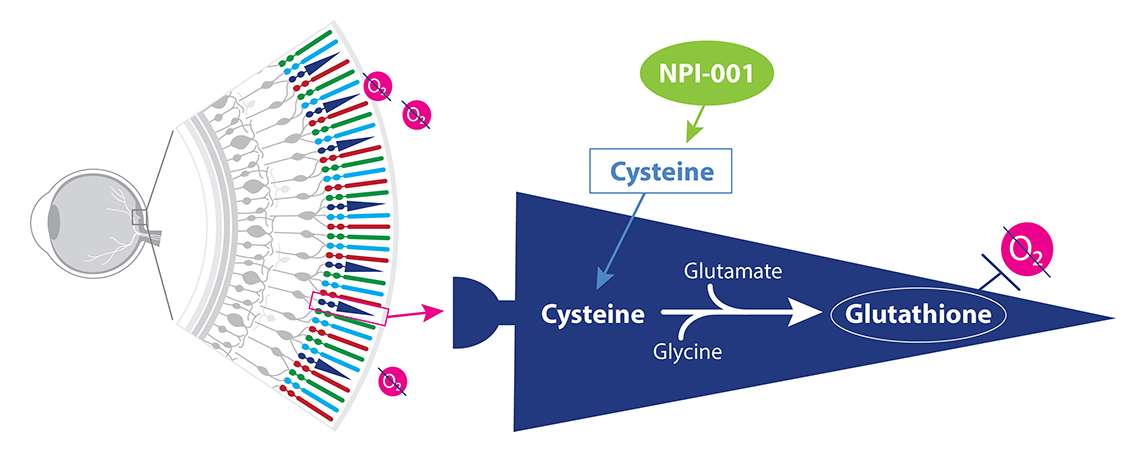
NPI-001 increases cysteine levels leading to a boost in glutathione.
Extensive preclinical studies of NPI-001 support a strong mechanistic rationale for its use in RP, in which oxidative stress plays a known role.
Dr. Campochiaro’s team demonstrated that orally administered NAC reduced cone cell death and preserved cone function by reducing oxidative damage in two models of RP, rd1+/+ and rd10+/+ mice.30
In addition, they compared N-acetylcysteine amide (NACA) with NAC in an animal model of RP and reported that mean photopic ERG b-wave amplitude was 50% higher (p=0.001) at all 3 stimulus intensities in NACA-treated versus NAC-treated mice, and more than 4-fold greater than controls.31 While mean peak scotopic ERG b-wave amplitude was higher in both NAC- or NACA-treated mice compared with controls, mean b-wave amplitudes were significantly greater in NACA-treated mice compared with NAC-treated mice at 10 of 11 stimulus intensities. Furthermore, cone cell density was significantly greater in 3 of 4 quadrants measured for NACA-treated compared to NAC-treated mice. Even with a substantially lower oral dose, NACA exhibited significantly greater preservation of cone cell function and survival compared with NAC in rd10+/+ mice.28, 9

Dr. Campochiaro’s work also explored NAC’s mechanism and its potential clinical use in RP. In a study of RP patients, Dr. Campochiaro’s team examined the safety, tolerability and visual function effects of oral NAC.32 Subjects (n = 10 per cohort) received 600 mg (cohort 1), 1200 mg (cohort 2), or 1800 mg (cohort 3) NAC twice a day for 12 weeks and then three times a day for 12 weeks. During the 24-week treatment period, mean best-corrected visual acuity significantly improved at 0.4 (95% CI: 0.2–0.6, P < 0.001), 0.5 (95% CI: 0.3–0.7, P > 0.001), and 0.2 (95% CI: 0.02–0.4, P = 0.03) letters/month in cohorts 1, 2, and 3, respectively. There was also a significant improvement in mean retinal sensitivity over time for cohort 3 (0.15 dB/month, 95% CI: 0.04–0.26). The results of this 24-week study of 30 subjects showed that oral NAC was relatively well-tolerated and suggested slight improvements in suboptimally functioning cones in patients with moderately advanced RP.
Nacuity seeks to build on this work and leverage NPI-001’s advantages over NAC.
NPI-001’s advantages as a therapy, based on preclinical models, include:
- Ability to cross blood-brain33 and blood-retinal barriers2
- Greater cell permeability than NAC34
- Approximately 3-fold more effective than oral NAC32, 31
NPI-001 oral tablets have been evaluated in a Phase 1/2 clinical trial (NCT04355689) in patients with RP associated with USH, demonstrating clinical proof of concept.
NP-2
Novel therapy for delay of cataract progression
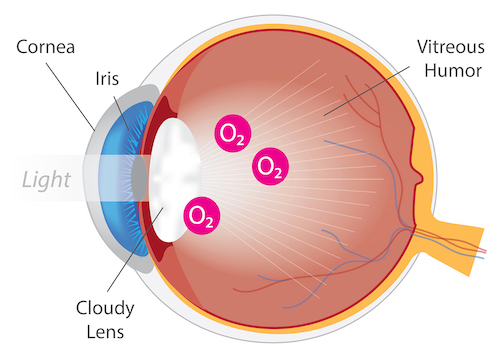
Eye with Cataract
Cataracts are the leading cause of blindness worldwide. A cataract, opacity or clouding of the eye lens, is a direct result of ocular oxidative damage. Symptoms of cataracts include blurry vision, double vision, glare and faded colors. While cataracts occur primarily due to age, they can also develop as a result of an injury or disease. For example, retinitis pigmentosa patients are at a greater risk for early cataract, and patients undergoing vitrectomy surgery exhibit accelerated cataract progression.
Currently, the only treatment for cataracts is surgical removal of the affected lens and replacement with an artificial, intraocular lens (IOL). Powerful antioxidants, including N-acetylcarnosine35 and N-acetylcysteine amide (NACA)11, 14 have been shown to prevent and/or treat cataracts in nonclinical models. No drugs are approved for the treatment of cataracts, resulting in significant unmet need and global market opportunity.
NPI-002 is a novel, differentiated, slow-release, small antioxidant molecule. In vitro and in vivo studies have revealed no cytotoxicity with NPI-002. In ex-vivo models with rat and porcine lenses, NPI-002 inhibited oxidative-induced cataracts.36 The novel intravitreal implant eluted NPI-002 in the vitreous humor in an animal model with no significant adverse findings. For the treatment of cataract, a sterile intravitreal implant containing NPI-002 has been developed, along with a precision delivery system. The NPI-002 implant is currently being assessed in a proof-of-concept Phase 1/2 clinical trial (NCT05026632).
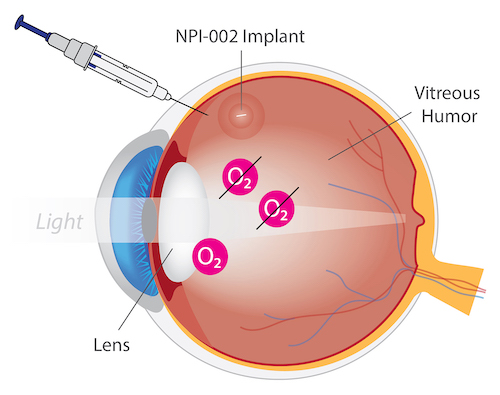
NPI-002 for Treatment of Cataracts
NP-3
Oral dosage for treatment of nephropathic cystinosis
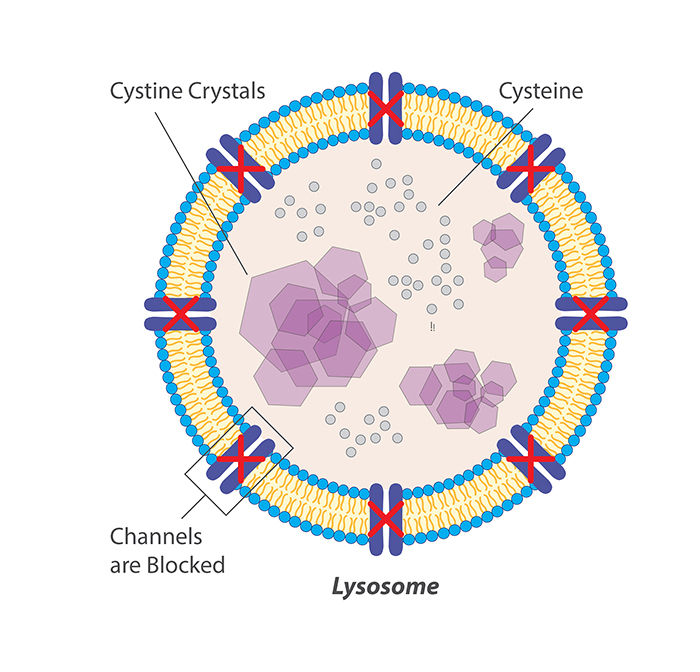
Cystinosis is a very rare autosomal lysosomal storage disease associated with high morbidity and mortality.37 Cystinosis leads to lysosomal cystine accumulation that can disrupt cellular structure and function. The most serious effect of cystine accumulation is often kidney dysfunction, which can be fatal.
All approved cystine-depleting drug products for cystinosis contain the aminothiol, cysteamine. Cysteamine treatment causes intralysosomal formation of a cysteamine–cysteine mixed disulfide, capable of exiting the lysosome purportedly via the lysine excretion pathway.38, 39 CYSTAGON® and PROCYSBI® are immediate- and delayed-release, respectively, oral dosage forms of cysteamine bitartrate. Oral administration of cysteamine, although efficacious for cystine depletion in nephropathic cystinosis, is associated with poor patient compliance due to the unpalatable taste, odor of sulfur, and gastrointestinal side-effects such as nausea, vomiting and pain, coupled with a requirement for frequent, high dosing to overcome extensive first pass metabolism of the cysteamine.40 Hence, there is an unmet medical need for alternative treatments for cystinosis.
Previous studies suggested that increased oxidative stress plays a role in the pathogenesis of the cystinosis.22, 19 Indeed, the antioxidant, N-acetylcysteine (NAC, the active ingredient in MUCOMYST®, ACETADOTE® and CETYLEV®) has been shown to improve cystinosis. In a 3-month study of 23 cystinosis patients less than 18 years old without renal replacement therapy, the addition of oral NAC 25 mg/kg/day to their cysteamine standard of care was associated with a reduction in oxidative stress (based on decreased serum thiobarbituric acid-reactive substances (TBARS) (p<0.0001)), and an improvement in renal function (based on levels of serum cystatin C, creatinine levels, and creatinine clearance).22
Nacuity has developed an oral dosage form to deliver NPI-001 for the treatment of nephropathic cystinosis. In a human cystinotic cell model, NPI-001 depleted cystine and increased cell viability significantly more than cysteamine.21 A chronic oral toxicology program has been successfully completed with NPI-001 along with a Phase 1 trial . A Phase 1/2 clinical trial in cystinosis patients is ongoing (NCT05994534).
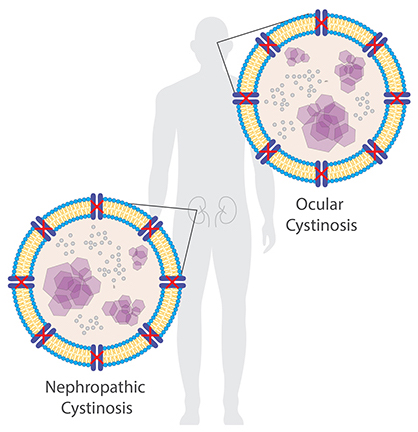
The accumulation of cystine crystals can damage many organs and tissues, especially the eyes and kidneys.
NP-4
Intravitreal implant for AMD and Stargardt disease
Age-related macular degeneration, commonly referred to as AMD, is a retinal degenerative disease that causes a progressive loss of central vision. AMD is the most common cause of blindness in individuals over the age of 55 in developed countries. More than 10 million people in the United States have AMD.16
Often diagnosed in childhood or adolescence, Stargardt disease is an inherited form of macular degeneration causing central vision loss. The condition is sometimes referred to as juvenile or early onset macular degeneration.16
Several therapies are now available for the wet form of AMD. Most involve regular ocular injections to halt the growth of leaky, vision-robbing blood vessels. These include: EYLEA™ (alflibercept), Lucentis™ (ranibizumab) and Avastin®.16
Schimel et al. (2011)17 independently demonstrated that N-acetylcysteine amide (NACA) remarkably protected retinal pigmented epithelium (RPE) and photoreceptors against oxidative cell damage and death, in vitro (ARPE-19 cell culture treated with tBHP) and in vivo (light damage mouse model). They concluded that NACA may be a novel treatment in rescuing retinal function and preventing vision loss secondary to retinal degenerative diseases including AMD.
A recent study18 was conducted to discern relative antioxidant effects of Nacuity’s antioxidants compared to N-acetylcysteine (NAC) in various retinal cell cultures. This study revealed that NPI-001, NPI-002, and NAC exhibited varying degrees of antioxidant activity, i.e., protected cultured rat retinal cells from a variety of stressors which were designed to mimic aspects of the pathology of different retinal diseases. A general rank order of activity was observed: NPI-001 ≥ NPI-002 > NAC. Studies are planned to further evaluate NPI-001 and NPI-002 as potential treatments for AMD and Stargardt disease.
NP-5
Oral dosage for treatment of rare neurological disease
Hereditary cystatin C amyloid angiopathy (HCCAA) is a rare, often fatal, neurological disease in Icelanders for which there is no approved therapy. The genetic deficiency in these patients leads to the formation of plaque that causes micro infarcts, stroke and often death in early adulthood. Nonclinical, in vitro proof of concept has been achieved for NPI-001 in HCCAA, with the demonstration that NPI-001 catabolizes L68Q-hCC-induced high molecular weight complexes that cause HCCA.22 Nacuity has developed an oral dosage form of NPI-001 for the treatment of HCCAA. Chronic oral toxicology studies and a Phase 1 clinical trial in healthy volunteers have been successfully completed. A clinical study, Safety, Tolerability and Efficacy of NPI-001 in Patients with Hereditary Cystatin C Amyloid Angiopathy (HCCAA), is ongoing in Iceland.

NPI-001 crosses the blood brain barrier as a potential treatment for a variety of neurological diseases.






















